Avoid these top 50 toxic ingredients in your skincare, cosmetics & supplements.
Shop our top recommended clean products.
Found in Beauty Products
Found in Supplements
Chemical Sunblockers
What are they: All UV-absorbing sunscreen ingredients other than Zinc Oxide and titanium dioxide.
Found in: Sunscreens, foundations, lipsticks, lotions, creams, moisturizers, concealers, and more.
Why you might not want to use these: Hormone disruption, increased risk of endometriosis, altered onset of puberty, lowered sperm count, increased percentage of abnormal sperm, altered birth weights, altered fetal development causing lasting problems, neurotoxicity, free radical production, phototoxicity, interference with thyroid function, promotion of obesity, and DNA damage potentially leading to cancer. 1-46
What names to look for on the bottle: Avobenzone, Cinoxate, Homosalate, Meradimate, Octisalate, Octinoxate, Octocrylene, Oxybenzone, PABA, Padimate O, Sulisobenzone, and all Benzophenones.
Actions to take: Choose sun protection (SPF) products which use Zinc Oxide and/or titanium dioxide. Avoid all substitutes.

Parabens
What are they: A type of preservative.
Found in: All types of facial and body cosmetics, as well as sunscreens, makeup remover, and even baby products such as diaper creams.
Why you might not want to use these: Adverse effects of parabens include increased risk of endometriosis, antiandrogenic activity, estrogenicity, endocrine disruption, DNA damage, increased cancer risk, and reproductive disorders. 47,65
What names to look for on the bottle: Methylparaben, Ethylparaben, Propylparaben, Butylparaben, Isobutylparaben, Isopropylparaben, and all names which include “paraben.”
Actions to take: Seek out paraben-free products. These safer products are available in most or all product categories.

PEG & Polysorbate Compounds
What are they: These are harmful ingredients used to help disperse (emulsify) poorly soluble cosmetic and supplement ingredients into water, whether in the product or in the digestive tract. These synthetic ingredients are produced by chemical reactions with ethylene oxide, a potent carcinogen.
Found in: Facial masks, moisturizers, mouthwashes, toothpaste, haircare products, lotions, gels, sunscreens, cleansers, and many other cosmetics types even including baby products. Polysorbates are also used as emulsifiers in nutritional supplements, particularly fish oil products.
Why you might not want to use these: In cosmetics, the principal reason to avoid these ingredients is that they may include residual ethylene oxide from the manufacturing process and may even contain 1,4-dioxane, another potent carcinogen.
In nutritional supplements, not only are these carcinogens a concern, but Polysorbate 80 and other synthetic emulsifiers have been shown to interfere greatly with the gut microbiome, the beneficial balance of baccteria in your intestinal tract which are shown to be of key importance to health. This occurs from the emulsifiers attacking the integrity of the gut mucosa. Adverse effects can include causing intestinal inflammation, inducing metabolic syndrome (pre-diabetic state), promoting obesity, and increasing anxiety.
Yet further, there is evidence polysorbates may increase risk of intestinal cancer.187-192.
What names to look for on the bottle: PEG-8, PEG-32, PEG-7 Glyceryl Cocoate, PEG-40 Stearate, Polysorbate, Polysorbate 80, Polysorbate 20, Polysorbate 40, Polysorbate 85, etc.
Actions to take: Avoid, particularly with oral products.

Phthalates
What are they: Solvents and plasticizers (materials which make plastic flexible instead of brittle.)
Found in: Fragrance (Parfum) used in cosmetics, though Fragrance does not always contain phthalates. They also commonly transferred into cosmetics, liquids, or foods from plastics.
Why you might not want to use these: Phthalates are associated with increased risk of endometriosis, obesity, increased waist circumference, insulin resistance, premature breast development in young girls, increased risk of autoimmune diseases, alterations of sexual development of male infants, reduction of female fertility, damage to sperm, behavior disruption in boys, and increased risk of breast cancer. 66-81
What names to look for on the bottle: Phthalates are rarely directly listed on ingredient labels but can come along with Fragrance (Parfum) or result from plasticizer contamination.
Actions to take: Choose fragrance-free cosmetics over products containing fragrance unless the manufacturers guarantees their products to be phthalate-free. Also, minimize contact of your food and beverages with plastics, and especially avoid using plastic containers when microwaving foods.

Perfluoro and Polyfluoro Compounds (PFAS’s)
What are they: Synthetic chemicals having multiple flourines.
Found in: Foundations, concealers, eyeliners, eye shadows, mascara, lipsticks, creams, moisturizers, and facial masks.
Why you might not want to use these: PFAS’s can interfere with thyroid function, worsen insulin sensitivity, promote diabetes, damage semen quality, promote obesity, and promote cancer. 82-89
What names to look for on the bottle: Perfluorononyl Dimethicone, Perfluorodecalin, Perfluorooctyl Triethoxysilane, Methyl Perfluorobutyl Ether, Perfluorohexane, and anything with polyflouro, pentafluoro, or octafluoro in the name.
Actions to take: Seek out cosmetics with no perfluoro ingredients, and try to avoid cosmetics containing Teflon, as it can carry perfluoro compounds as contaminants.

Retinol and Retinyl Compounds
What are they: Vitamin A derivatives, often used for antiwrinkle or skin-thinning effect but often merely to meet market demand.
Found in: Foundations, lipstick, Serums, moisturizers, concealers, creams, masks, body oils, body lotions, blushes, bronzers, eyeliner, mascara, and even baby products.
Why you might not want to use these:
Retinol and retinyl compounds are unstable to sun and can promote cancer when exposed to sun. Also although less importantly, they commonly cause adverse effects of dryness, itching, redness, burning, scaling, peeling, and hives. 90-96
What names to look for on the bottle: Retinol, retinyl palmitate, and all ingredients with retinyl in their names.
Actions to take: Avoid cosmetics which use retinol or retinyl compounds. Use such ingredients only under dermatological supervision and with full understanding of the risks and needed precautions. Cosmetics using bakuchiol can be safer alternatives to retinol-based products.

Formaldehyde and Formaldehyde Releasers
What are they: Preservatives and hair straighteners
Found in: Hair straighteners, shampoos and hair conditioners, moisturizers, foundations, lipsticks, facial powders, mascara, lotions, creams, cleansers, body washes, nail treatments, makeup remover, and nearly all types of cosmetics.
Why you might not want to use these: Formaldehyde is carcinogenic, a potent skin allergen, and toxic to the lungs. 97-108
What names to look for on the bottle: Formaldehyde, formalin, formic aldehyde, methanediol, methanal, methyl aldehyde, methylene glycol, methylene oxide, bonded aldehyde, morbicid acid, DMDM Hydantoin, Diazolidinyl Urea, Imidazolidinyl Urea, Sodium Hydroxymethylglycinate, Quaternium-15, Bronopol (2-Bromo-2-Nitropropane-1,3-Diol), Glyoxal, and Polyoxymethylene Urea.
Actions to take: If you feel a hair straightener is needed and do not wish to use a lye-based straightener, unfortunately the only other effective product type is formaldehyde based. If choosing to use such a product, take great care to provide excellent ventilation and to prevent skin contact. With regard to all other product types, simply avoid these ingredients as they are unnecessary to properly formulated products and are toxic.

Methylchloroisothiazolinone, Methylisothiazolinone, and Benzisothiazolinone
What are they: Preservatives.
Found in: Over 1000 products in the US from brands such as but not limited to Aveno, Aussie, Dove, L’Oreal, Neutrogena, Nexus, Pantene, Paul Mitchell, Suave, and TRESemmé, in nearly every category including baby products. They are also found in many cleaning products which may contact the skin.
Why you might not want to use these: These thiazols are potent allergic sensitizers which can cause hives, scaling, burning, and blistering of skin, and are neurotoxic (potentially causing damage to the brain or nervous system). They are banned in Europe for leave-on products. 117-126
What names to look for on the bottle: Methylchloroisothiazolinone, Methylisothiazolinone, and Benzisothiazolinone.
Actions to take: Find different products not containing these. No product needs these ingredients to be properly preserved.

Ethanolamines and Ethanolamine Derivatives
What are they: Materials used to increase alkalinity or to provide detergent-like action.
Found in: Nearly all types of cosmetics.
Why you might not want to use these: Ethanolamine and diethanolamine react with other compounds, air pollution, or materials in bottles or seals to produce nitrosamines which are potent carcinogens. Triethanolamine and ethanolamine derivatives with names having MEA, DEA, and TEA can include these as unlisted ingredients and so can be toxic as well. 127-130
What names to look for on the bottle: Ethanolamine, diethanolamine, triethanolamine, and any compounds with names starting or ending with DEA or TEA, such as Acetamide MEA, Cocamide DEA, and TEA Laureth Sulfate.
Actions to take: Seek out products lacking these ingredients. If however you feel a need to choose between products containing any of these these ingredients, MEA and TEA compounds are less prone to producing carcinogenic nitrosamines than are DEA compounds or ethanolamine.

BHA (Butylated Hydroxyanisole)
What is this: An antioxidant to extend shelf life of products.
Found in: Lipsticks, facial products, and diaper creams.
Why you might not want to use this: BHA (butylated hydroxyanisole) is a proven carcinogen. Further, it is an endocrine disruptor adversely affecting the functions of both estrogen and testosterone and it can interfere with testicular function. Further, it is neurotoxic. 131-136
What names to look for on the bottle: BHA
Actions to take: Avoid.

Allergic Sensitizers and Synthetic Colors
What are they: Ingredients which can cause not only an immediate allergic reaction but lasting extreme sensitivity to themselves and to other ingredients. These include but are not limited to dyes, preservatives, and fragrance materials.
Found in: Permanent hair dyes, and many colored or fragranced cosmetics.
Why you might not want to use these: There are two types of irritation that can occur when ingredients are applied to your skin in greater concentration than you personally can handle. The first, irritant contact dermatitis, is not severe. Your skin has an immediate or quick reaction to such as a rash, occurring only at the area of application. In this type of reaction, as soon your skin heals, your sensitivity to that ingredient is no higher than it was before, nor is your sensitivity to anything else. The second reaction type, allergic contact dermatitis, is a different story. Your body can permanently become completely intolerant not only of that ingredient, but many others as well, often with severe reactions. The reaction can occur in areas other than application as well, and can extend far beyond rash, even to inability to breathe and (rarely) death. Unfortunately, there is no way to predict who is susceptible and who is not. About 10% of Americans are allergic to at least one common cosmetic ingredient. 137-148
What names to look for on the bottle: Basic Violet 10, Basic Violet 3 (Gentian Violet), D&C Colors, Fragrance (Parfum), Phenylenediamine, Resorcinol, Toluene-2,5-diamine. Many more ingredients can cause allergic reactions but these are the most common and severe culprits.
As for other synthetic colorants, including the FD&C Colors, the very nature of molecules that can interact with light as colorants inherently creates potential for undesired reactions. Further, some of the FD&C dyes can have toxic contaminants such as mercury, 6-methoxy-m-toluidine, aniline, and cadmium. Further, the chemical environment of permanent hair colors inherently can lead to conversion (nitrososation) of many synthetic dyes into carcinogens. For this reason, permanent hair dyes generally should not be allowed to touch human skin.
Actions to take: When choosing to use a product having unfamiliar or unknown ingredients, first apply a small amount to an area of the skin such as near the crook of the elbow and apply a bandaid-type covering. Evaluate 48-72 hours later. If irritation is felt anytime before then, remove and inspect the area. If any problem such as a rash appears, thoroughly wash the applied area, and do not use that product.

Unlisted Contaminants (Pesticides, Manufacturing Byproducts, etc.)
What are they: These are harmful ingredients which enter the product as unlisted impurities, some of which are toxic. Manufacturers only have to list what their ingredients are supposed to be and are not required to state whether those ingredients are free of these toxic contaminants.
Some of these toxic contaminants are created as a byproduct of the product’s manufacturing process. For example, sodium laureth sulfate (the “eth” denotes ethoxylation) is a modified version of sodium lauryl sulfate. The intent of the modification was to make it less harsh, but the ethoxylation process readily generates the contaminant 1,4-dioxane, a carcinogen linked to organ toxicity.
Found in: Potentially present in nearly any type of cosmetics and in nutritional supplements.
Why you might not want to use these: The most serious concerns of unlisted contaminants are cancer and endocrine disruption, but potentially many other adverse effects could occur. 148-154
What names to look for on the bottle: Ethoxylated ingredients, which include everything with names such as Sodium Laureth Sulfate, Ceteareth-20, and Steareth-2, as well as all PEG and Polysorbate ingredients can potentially be contaminated with carcinogenic 1,4-dioxane and ethylene oxide. Products having ethanolamine-type ingredients such as ethanolamine itself, diethanolamine, triethanolamine, and the abbreviated MEA and DEA variants such as Cocamide MEA and Lauramide DEA can be contaminated with carcinogenic nitrosamines. Talc can be contaminated with carcinogenic asbestos. Ingredients including “acrylate” within them can be contaminated with acrylates or methacrylates. Further, products can be contaminated with lead, cadmium, arsenic, mercury, and/or pesticides without any clue being available on the label.
Actions to take: Evaluate for yourself the trustworthiness of each manufacturer. Ideally, choose products which have undergone third-party safety testing. Famous brand names on products, unfortunately, in many cases mean nothing as to trustworthiness.

Triclosan
What is this: An antimicrobial ingredient and preservative.
Found in: Soaps, toothpastes, and sanitizers, but is rapidly being removed from the market.
Why you might not want to use this: Triclosan is a potent disruptor of the endocrine system. Specific adverse effects which triclosan can cause include damaged sperm quality, reduced female fertility, abnormal menstruation, reduced bone mass, increased risk of osteoporosis, thyroid inhibition, autoimmune attack of the thyroid, damage to the microbiome of nursing infants, and earlier breast development in young girls.109-116
What names to look for on the bottle: Triclosan.
Actions to take: Strictly avoid.

Toxic Ingredients Found in Supplements
Artificial Colors
What are they: Synthetic ingredients used to change color of products.
Found in: Most supplement types, with the largest amounts of artificial colors commonly appearing in beverages and powders intended to be mixed with water.
Why you might not want to use these: Consumption of artificial food colors have been linked in multiple studies to increased rate of ADHD and autism in children, and may promote immune disorders. 155-157
What names to look for on the bottle: Red 40, Red 3, Yellow 5, Yellow 6, Blue 1, Blue 2, and Green 3. These may be written as, for example, Red No. 40, etc.
Actions to take: Preferably avoid, particularly for children.

Hydrogenated Oils
What are they: Oils subjected to a chemical reaction intended to convert double bonds as found in unsaturated fats to single bonds as found in saturated fats. This can give the oils a longer shelf life, a higher smoke point, or make them solid at room temperature.
Found in: Softgels, meal replacement drinks and powders, and foods.
Why you might not want to use these: The hydrogenation process produces trans fats as a side product. Consumption of trans fats increases risk of heart disease by increasing LDL-C (a component of “bad cholesterol” and increases rate of death from heart disease. 158-165
What names to look for on the bottle: Any oil with the word “hydrogenated,” e.g. hydrogenated palm oil.
Actions to take: Avoid.

Lead, Mercury, and PCB’s
What are they: Contaminants which will not be listed on a label but may be present.
Found in: PCB’s (polychlorinated biphenyls) and mercury are found particularly in marine products such as fish oils, algae products, and seaweed products. Lead can potentially be a trace contaminant in many product types but calcium products are perhaps the most likely to have major contamination. 166-175
Why you might not want to use these: Lead or mercury exposure can severely affect the nerves of the brain and adversely affect psychiatric function, and can harm the intellectual development of children. Further, lead exposure can promote cancer and adversely affect female reproductive health. PCB’s are liver toxic, are toxins to the reproductive system, may promote atherosclerosis and other inflammatory diseases, and are carcinogenic. 176-185
What names to look for on the bottle:These contaminants will not be listed.
Actions to take: To be sure of avoiding these dangerous contaminants, purchase from suppliers whose products undergo third party testing.

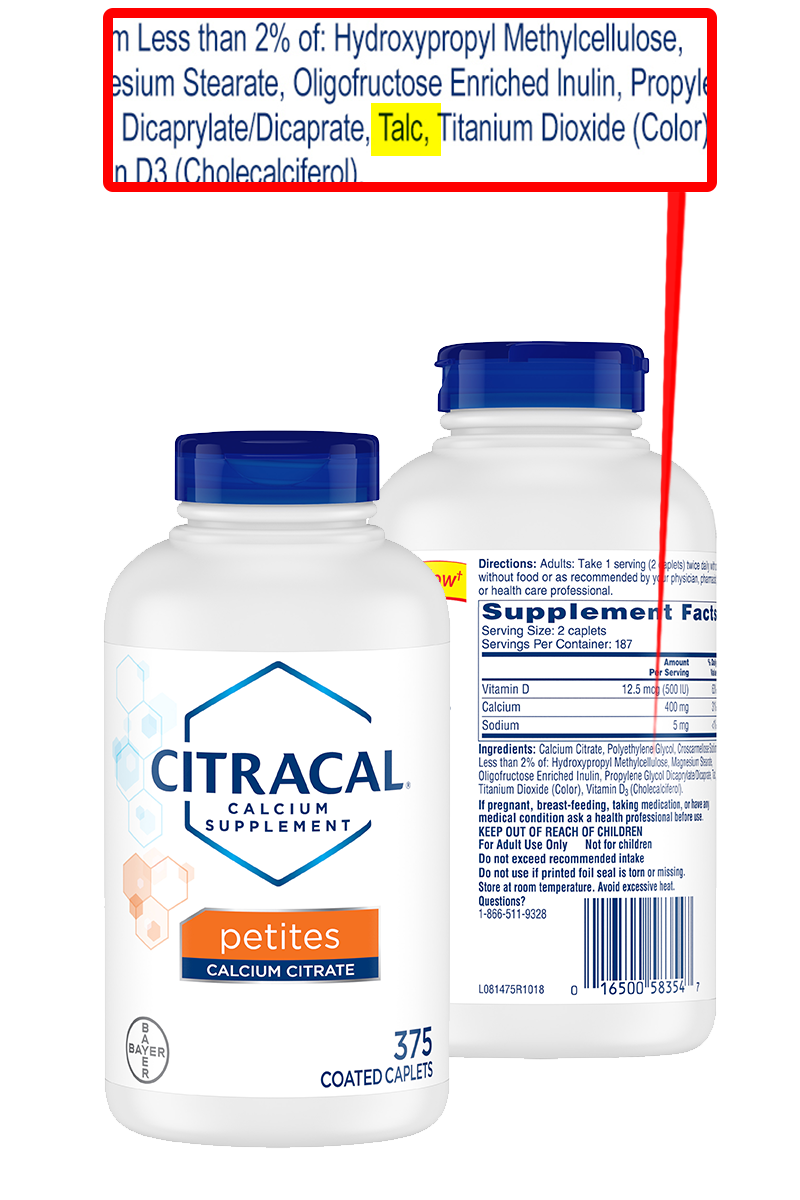
Talc (Magnesium Silicate)
What is this: A powder which can improve flowability of powdered products.
Found in: Potentially in any capsule product.
Why you might not want to use this: Talc can be contaminated with asbestos, which is carcinogenic.154
What names to look for on the bottle: Talc or magnesium silicate.
Actions to take: Preferably avoid.
Titanium Dioxide (Oral)
What is this: A material added to increase whiteness of appearance of supplements.
Found in: Nutritional supplement powders.
Why you might not want to use this: Titanium dioxide taken orally can cause inflammation in the small intestine.187
Titanium oxide is safe for skin application, but take care to avoid oral ingestion even of trace amounts. Such ingestion can readily occur from sunscreen use if particular care is not taken.
What names to look for on the bottle: Titanium dioxide or titanium oxide.
Actions to take: Preferably avoid.

References
- Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35(3):424-436. doi:10.1111/j.1365-2605.2012.01280.x
- Wang J, Pan L, Wu S, et al. Recent Advances on Endocrine Disrupting Effects of UV Filters. Int J Environ Res Public Health. 2016;13(8):782. Published 2016 Aug 3. doi:10.3390/ijerph13080782
- Song S, He Y, Huang Y, et al. Occurrence and transfer of benzophenone-type ultraviolet filters from the pregnant women to fetuses. Sci Total Environ. 2020;726:138503. doi:10.1016/j.scitotenv.2020.138503
- Hayashi T, Okamoto Y, Ueda K, Kojima N. Formation of estrogenic products from benzophenone after exposure to sunlight. Toxicol Lett. 2006;167(1):1-7. doi:10.1016/j.toxlet.2006.08.001
- Klopcic I, Dolenc MS. Endocrine Activity of AVB, 2MR, BHA, and Their Mixtures. Toxicol Sci. 2017;156(1):240-251. doi:10.1093/toxsci/kfw253
- Takemoto K, Yamazaki H, Nakajima M, Yokoi T. Genotoxic activation of benzophenone and its two metabolites by human cytochrome P450s in SOS/umu assay. Mutat Res. 2002;519(1-2):199-204. doi:10.1016/s1383-5718(02)00141-9
- Rehfeld A, Egeberg DL, Almstrup K, Petersen JH, Dissing S, Skakkebæk NE. EDC IMPACT: Chemical UV filters can affect human sperm function in a progesterone-like manner. Endocr Connect. 2018;7(1):16-25. doi:10.1530/EC-17-0156
- Jiménez-Díaz I, Molina-Molina JM, Zafra-Gómez A, et al. Simultaneous determination of the UV-filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC-MS/MS. Assessment of their in vitro endocrine activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;936:80-87. doi:10.1016/j.jchromb.2013.08.006
- Lorigo M, Mariana M, Cairrao E. Photoprotection of ultraviolet-B filters: Updated review of endocrine disrupting properties. Steroids. 2018;131:46-58. doi:10.1016/j.steroids.2018.01.006
- Williams GP, Darbre PD. Low-dose environmental endocrine disruptors, increase aromatase activity, estradiol biosynthesis and cell proliferation in human breast cells. Mol Cell Endocrinol. 2019;486:55-64. doi:10.1016/j.mce.2019.02.016
- Ma R, Cotton B, Lichtensteiger W, Schlumpf M. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74(1):43-50. doi:10.1093/toxsci/kfg102
- Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol Appl Pharmacol. 2005;203(1):9-17. doi:10.1016/j.taap.2004.07.005
- Kunisue T, Chen Z, Buck Louis GM, et al. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ Sci Technol. 2012;46(8):4624-4632. doi:10.1021/es204415a
- Peinado FM, Ocón-Hernández O, Iribarne-Durán LM, et al. Cosmetic and personal care product use, urinary levels of parabens and benzophenones, and risk of endometriosis: results from the EndEA study [published online ahead of print, 2020 Oct 16]. Environ Res. 2020;110342. doi:10.1016/j.envres.2020.110342
- Huang Y, Wang P, Law JC, et al. Organic UV filter exposure and pubertal development: A prospective follow-up study of urban Chinese adolescents. Environ Int. 2020;143:105961. doi:10.1016/j.envint.2020.105961
- Harley KG, Berger KP, Kogut K, et al. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum Reprod. 2019;34(1):109-117. doi:10.1093/humrep/dey337
- Buck Louis GM, Chen Z, Kim S, Sapra KJ, Bae J, Kannan K. Urinary concentrations of benzophenone-type ultraviolet light filters and semen quality. Fertil Steril. 2015;104(4):989-996. doi:10.1016/j.fertnstert.2015.07.1129
- Ghazipura M, McGowan R, Arslan A, Hossain T. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod Toxicol. 2017;73:175-183. doi:10.1016/j.reprotox.2017.08.015
- Krause M, Frederiksen H, Sundberg K, et al. Maternal exposure to UV filters: associations with maternal thyroid hormones, IGF-I/IGFBP3 and birth outcomes. Endocr Connect. 2018;7(2):334-346. doi:10.1530/EC-17-0375 (also thyroid)
- Ferguson KK, Meeker JD, Cantonwine DE, et al. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int. 2018;112:243-250. doi:10.1016/j.envint.2017.12.011 (also triclosan)
- Tang R, Chen MJ, Ding GD, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115-120. doi:10.1016/j.envpol.2013.03.023
- Long J, Xia W, Li J, et al. Maternal urinary benzophenones and infant birth size: Identifying critical windows of exposure. Chemosphere. 2019;219:655-661. doi:10.1016/j.chemosphere.2018.11.190
- Aker AM, Ferguson KK, Rosario ZY, et al. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ Res. 2019;169:41-51. doi:10.1016/j.envres.2018.10.030 (duration)
- Wnuk A, Rzemieniec J, Staroń J, et al. Prenatal Exposure to Benzophenone-3 Impairs Autophagy, Disrupts RXRs/PPARγ Signaling, and Alters Epigenetic and Post-Translational Statuses in Brain Neurons. Mol Neurobiol. 2019;56(7):4820-4837. doi:10.1007/s12035-018-1401-5
- Jiang Y, Zhao H, Xia W, et al. Prenatal exposure to benzophenones, parabens and triclosan and neurocognitive development at 2 Environ Int. 2019;126:413-421. doi:10.1016/j.envint.2019.01.023
- Guo J, Wu C, Zhang J, et al. Maternal and childhood urinary phenol concentrations, neonatal thyroid function, and behavioral problems at 10 years of age: The SMBCS study. Sci Total Environ. 2020;743:140678. doi:10.1016/j.scitotenv.2020.140678
- Hsieh MH, Grantham EC, Liu B, Macapagal R, Willingham E, Baskin LS. In utero exposure to benzophenone-2 causes hypospadias through an estrogen receptor dependent mechanism. J Urol. 2007;178(4 Pt 2):1637-1642. doi:10.1016/j.juro.2007.03.190
- Ruszkiewicz JA, Pinkas A, Ferrer B, Peres TV, Tsatsakis A, Aschner M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol Rep. 2017;4:245-259. Published 2017 May 27. doi:10.1016/j.toxrep.2017.05.006
- Amar SK, Goyal S, Srivastav AK, Chopra D, Ray RS. Combined effect of Benzophenone-2 and ultraviolet radiation promote photogenotoxicity and photocytotoxicity in human keratinocytes [published correction appears in Regul Toxicol Pharmacol. 2019 Feb;101:201-202]. Regul Toxicol Pharmacol. 2018;95:298-306. doi:10.1016/j.yrtph.2018.04.003
- Placzek M, Dendorfer M, Przybilla B, Gilbertz KP, Eberlein B. Photosensitizing properties of compounds related to benzophenone. Acta Derm Venereol. 2013;93(1):30-32. doi:10.2340/00015555-1421
- Amar SK, Goyal S, Dubey D, et al. Benzophenone 1 induced photogenotoxicity and apoptosis via release of cytochrome c and Smac/DIABLO at environmental UV radiation. Toxicol Lett. 2015;239(3):182-193. doi:10.1016/j.toxlet.2015.09.024
- Amar SK, Goyal S, Mujtaba SF, et al. Role of type I & type II reactions in DNA damage and activation of caspase 3 via mitochondrial pathway induced by photosensitized benzophenone [published correction appears in Toxicol Lett. 2019 Oct 10;314:194-195]. Toxicol Lett. 2015;235(2):84-95. doi:10.1016/j.toxlet.2015.03.008
- Avenel-Audran M, Dutartre H, Goossens A, et al. Octocrylene, an emerging photoallergen. Arch Dermatol. 2010;146(7):753-757. doi:10.1001/archdermatol.2010.132
- Karlsson I, Vanden Broecke K, Mårtensson J, Goossens A, Börje A. Clinical and experimental studies of octocrylene's allergenic potency. Contact Dermatitis. 2011;64(6):343-352. doi:10.1111/j.1600-0536.2011.01899.x
- Gunia-Krzyżak A, Słoczyńska K, Popiół J, Koczurkiewicz P, Marona H, Pękala E. Cinnamic acid derivatives in cosmetics: current use and future prospects. Int J Cosmet Sci. 2018;40(4):356-366. doi:10.1111/ics.12471
- Gaspar LR, Tharmann J, Maia Campos PM, Liebsch M. Skin phototoxicity of cosmetic formulations containing photounstable and photostable UV-filters and vitamin A palmitate. Toxicol In Vitro. 2013;27(1):418-425. doi:10.1016/j.tiv.2012.08.006
- Benevenuto CG, Guerra LO, Gaspar LR. Combination of retinyl palmitate and UV-filters: phototoxic risk assessment based on photostability and in vitro and in vivo phototoxicity assays. Eur J Pharm Sci. 2015;68:127-136. doi:10.1016/j.ejps.2014.12.007
- Greenspoon J, Ahluwalia R, Juma N, Rosen CF. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24(1):29-32. doi:10.1097/DER.0b013e31827edc8b
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37(5):221-232. doi:10.1111/j.1600-0536.1997.tb02439.x
- Kaidbey KH, Kligman AM. Phototoxicity to a sunscreen ingredient. Padimate A. Arch Dermatol. 1978;114(4):547-549.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25(6):289-326. doi:10.1097/DER.0000000000000079
- Shin JC, Lee E, An S, et al. Benzophenone-3 and benzophenone-8 exhibit obesogenic activity via peroxisome proliferator-activated receptor γ Toxicol In Vitro. 2020;67:104886. doi:10.1016/j.tiv.2020.104886
- Ahn S, An S, Lee M, et al. A long-wave UVA filter avobenzone induces obesogenic phenotypes in normal human epidermal keratinocytes and mesenchymal stem cells. Arch Toxicol. 2019;93(7):1903-1915. doi:10.1007/s00204-019-02462-1
- Knowland J, McKenzie EA, McHugh PJ, Cridland NA. Sunlight-induced mutagenicity of a common sunscreen ingredient. FEBS Lett. 1993;324(3):309-313. doi:10.1016/0014-5793(93)80141-g
- Cuquerella MC, Lhiaubet-Vallet V, Cadet J, Miranda MA. Benzophenone photosensitized DNA damage. Acc Chem Res. 2012;45(9):1558-1570. doi:10.1021/ar300054e
- Shimoi K, Nakamura Y, Noro T, et al. Enhancing effects of cinoxate and methyl sinapate on the frequencies of sister-chromatid exchanges and chromosome aberrations in cultured mammalian cells. Mutat Res. 1989;212(2):213-221. doi:10.1016/0027-5107(89)90072-9
- Darbre, P.D. and Harvey, P.W. (2008), Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. Appl. Toxicol., 28: 561-578. https://doi.org/10.1002/jat.1358
- Nishihama Y, Yoshinaga J, Iida A, et al. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol. 2016;63:107-113. doi:10.1016/j.reprotox.2016.05.010
- Lucaccioni L, Trevisani V, Marrozzini L, et al. Endocrine-Disrupting Chemicals and Their Effects during Female Puberty: A Review of Current Evidence. Int J Mol Sci. 2020;21(6):2078. Published 2020 Mar 18. doi:10.3390/ijms21062078
- Darbre, P. D. and Harvey, P. W. (2014), Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: a review of the literature with reference to new exposure data and regulatory status, Appl. Toxicol., 34, pages 925– 938. DOI:10.1002/jat.3027
- Lillo MA, Nichols C, Perry C, et al. Methylparaben stimulates tumor initiating cells in ER+ breast cancer models. J Appl Toxicol. 2017;37(4):417-425. doi:10.1002/jat.3374
- Pan S, Yuan C, Tagmount A, et al. Parabens and Human Epidermal Growth Factor Receptor Ligand Cross-Talk in Breast Cancer Cells. Environ Health Perspect. 2016;124(5):563-569. doi:10.1289/ehp.1409200
- Majhi PD, Sharma A, Roberts AL, et al. Effects of Benzophenone-3 and Propylparaben on Estrogen Receptor-Dependent R-Loops and DNA Damage in Breast Epithelial Cells and Mice. Environ Health Perspect. 2020;128(1):17002. doi:10.1289/EHP5221
- Gonzalez TL, Moos RK, Gersch CL, et al. Metabolites of n-Butylparaben and iso-Butylparaben Exhibit Estrogenic Properties in MCF-7 and T47D Human Breast Cancer Cell Lines. Toxicol Sci. 2018;164(1):50-59. doi:10.1093/toxsci/kfy063
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect. 2011;119(2):252-257. doi:10.1289/ehp.1002238
- Zhang L, Ding S, Qiao P, et al. n-butylparaben induces male reproductive disorders via regulation of estradiol and estrogen receptors. J Appl Toxicol. 2016;26(9):1223-1234. doi:10.1002/jat.3291
- Özdemir E, Barlas N, Çetinkaya MA. Assessing the antiandrogenic properties of propyl paraben using the Hershberger bioassay. Toxicol Res (Camb). 2018;7(2):235-243. Published 2018 Jan 25. doi:10.1039/c7tx00319f
- Smarr MM, Sundaram R, Honda M, Kannan K, Louis GM. Urinary Concentrations of Parabens and Other Antimicrobial Chemicals and Their Association with Couples' Fecundity. Environ Health Perspect. 2017;125(4):730-736. doi:10.1289/EHP189
- Pollack AZ, Mumford SL, Krall JR, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach. Environ Int. 2018;120:137-144. doi:10.1016/j.envint.2018.07.028
- Guo J, Wu C, Lu D, et al. Urinary paraben concentrations and their associations with anthropometric measures of children aged 3 years. Environ Pollut. 2017;222:307-314. doi:10.1016/j.envpol.2016.12.040
- Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol Metab. 2018;29(9):607-625. doi:10.1016/j.tem.2018.06.003
- Aker AM, Watkins DJ, Johns LE, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30-37. doi:10.1016/j.envres.2016.07.002
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int. 2018;113:341-349. doi:10.1016/j.envint.2018.01.006
- Berger K, Gunier RB, Chevrier J, et al. Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ Res. 2018;165:379-386. doi:10.1016/j.envres.2018.05.005
- Geer LA, Pycke BFG, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 2017;323(Pt A):177-183. doi:10.1016/j.jhazmat.2016.03.028
- Dutta S, Haggerty DK, Rappolee DA, Ruden DM. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front Genet. 2020;11:405. Published 2020 May 6. doi:10.3389/fgene.2020.00405
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293-342. doi:10.1210/er.2009-0002
- Popescu M, Feldman TB, Chitnis T. Interplay Between Endocrine Disruptors and Immunity: Implications for Diseases of Autoreactive Etiology. Front Pharmacol. 2021;12:626107. Published 2021 Mar 23. doi:10.3389/fphar.2021.626107
- Colón I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108(9):895-900. doi:10.1289/ehp.108-2556932
- Chiang C, Mahalingam S, Flaws JA. Environmental Contaminants Affecting Fertility and Somatic Health. Semin Reprod Med. 2017;35(3):241-249. doi:10.1055/s-0037-1603569
- Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056-1061. doi:10.1289/ehp.8100
- Grün F. Obesogens. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):453-459. doi:10.1097/MED.0b013e32833ddea0
- Hatch EE, Nelson JW, Qureshi MM, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27. Published 2008 Jun 3. doi:10.1186/1476-069X-7-27
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876-882. doi:10.1289/ehp.9882
- Nazir S, Usman Z, Imran M, Lone KP, Ahmad G. Women Diagnosed with Endometriosis Show High Serum Levels of Diethyl Hexyl Phthalate. J Hum Reprod Sci. 2018;11(2):131-136. doi:10.4103/jhrs.JHRS_137_17
- Calaf GM, Ponce-Cusi R, Aguayo F, Muñoz JP, Bleak TC. Endocrine disruptors from the environment affecting breast cancer. Oncol Lett. 2020;20(1):19-32. doi:10.3892/ol.2020.11566
- De Falco M, Forte M, Laforgia V. Estrogenic and anti-androgenic endocrine disrupting chemicals and their impact on the male reproductive system. Front Environ Sci (2015) 3:3.10.3389/fenvs.2015.00003
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682-691. doi:10.1097/01.ede.0000235996.89953.d7
- Hauser R, Meeker JD, Singh NP, et al. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22(3):688-695. doi:10.1093/humrep/del428
- Smarr MM, Kannan K, Sun L, et al. Preconception seminal plasma concentrations of endocrine disrupting chemicals in relation to semen quality parameters among male partners planning for pregnancy. Environ Res. 2018;167:78-86. doi:10.1016/j.envres.2018.07.004
- Philippat C, Nakiwala D, Calafat AM, et al. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ Health Perspect. 2017;125(9):097014. Published 2017 Sep 15. doi:10.1289/EHP1314
- Temkin AM, Hocevar BA, Andrews DQ, Naidenko OV, Kamendulis LM. Application of the Key Characteristics of Carcinogens to Per and Polyfluoroalkyl Substances. Int J Environ Res Public Health. 2020;17(5):1668. Published 2020 Mar 4. doi:10.3390/ijerph17051668
- Ministry of Environment and Food of Denmark. Risk assessment of fluorinated substances in cosmetic products. Survey of chemical substances in consumer products No. 169, October 2018, ISBN: 978-87-93710-94-8 https://www2.mst.dk/Udgiv/publications/2018/10/978-87-93710-94-8.pdf
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118(5):686-692. doi:10.1289/ehp.0901584
- Qi W, Clark JM, Timme-Laragy AR, Park Y. Per- and Polyfluoroalkyl Substances and Obesity, Type 2 Diabetes and Non-alcoholic Fatty Liver Disease: A Review of Epidemiologic Findings. Toxicol Environ Chem. 2020;102(1-4):1-36. doi:10.1080/02772248.2020.1763997
- Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120(5):668-673. doi:10.1289/ehp.1104034
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jørgensen N. Do perfluoroalkyl compounds impair human semen quality?. Environ Health Perspect. 2009;117(6):923-927. doi:10.1289/ehp.0800517
- Du G, Hu J, Huang H, et al. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem. 2013;32(2):353-360. doi:10.1002/etc.2034
- White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127(1-2):16-26. doi:10.1016/j.jsbmb.2011.03.011
- Fu PP, Cheng SH, Coop L, et al. Photoreaction, phototoxicity, and photocarcinogenicity of retinoids. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003;21(2):165-197. doi:10.1081/GNC-120026235
- Tolleson WH, Cherng SH, Xia Q, et al. Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health. 2005;2(1):147-155. doi:10.3390/ijerph2005010147
- Yan J, Xia Q, Cherng SH, et al. Photo-induced DNA damage and photocytotoxicity of retinyl palmitate and its photodecomposition products. Toxicol Ind Health. 2005;21(7-8):167-175. doi:10.1191/0748233705th225oa
- Kryczyk-Poprawa A, Kwiecień A, Opoka W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics. 2019;12(1):10. Published 2019 Dec 20. doi:10.3390/pharmaceutics12010010
- Boudreau MD, Beland FA, Felton RP, et al. Photo-co-carcinogenesis of Topically Applied Retinyl Palmitate in SKH-1 Hairless Mice. Photochem Photobiol. 2017;93(4):1096-1114. doi:10.1111/php.12730
- Mei N, Xia Q, Chen L, Moore MM, Chen T, Fu PP. Photomutagenicity of anhydroretinol and 5,6-epoxyretinyl palmitate in mouse lymphoma cells. Chem Res Toxicol. 2006;19(11):1435-1440. doi:10.1021/tx0600907
- Mei N, Xia Q, Chen L, Moore MM, Fu PP, Chen T. Photomutagenicity of retinyl palmitate by ultraviolet A irradiation in mouse lymphoma cells. Toxicol Sci. 2005;88(1):142-149. doi:10.1093/toxsci/kfi291
- Blackwell M, Kang H, Thomas A, Infante P. Formaldehyde: evidence of carcinogenicity. Am Ind Hyg Assoc J. 1981;42(7)
- Yu R, Lai Y, Hartwell HJ, et al. Formation, Accumulation, and Hydrolysis of Endogenous and Exogenous Formaldehyde-Induced DNA Damage. Toxicol Sci. 2015;146(1):170-182. doi:10.1093/toxsci/kfv079
- Chappell G, Pogribny IP, Guyton KZ, Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat Res Rev Mutat Res. 2016;768:27-45. doi:10.1016/j.mrrev.2016.03.004
- Solomons K, Cochrane JW. Formaldehyde toxicity. Part II. Review of acute and chronic effects on health. S Afr Med J. 1984;66(3):103-106.
- Malinauskiene L, Blaziene A, Chomiciene A, Isaksson M. Formaldehyde may be found in cosmetic products even when unlabelled. Open Med (Wars). 2015;10(1):323-328. Published 2015 Jul 7. doi:10.1515/med-2015-0047
- Voller LM, Persson L, Bruze M, Ericson ME, Hylwa SA. Formaldehyde in "Nontoxic" Nail Polish. Dermatitis. 2019;30(4):259-263. doi:10.1097/DER.0000000000000493
- Uter W, Werfel T, White IR, Johansen JD. Contact Allergy: A Review of Current Problems from a Clinical Perspective. Int J Environ Res Public Health. 2018;15(6):1108. Published 2018 May 29. doi:10.3390/ijerph15061108
- de Groot AC, Veenstra M. Formaldehyde-releasers in cosmetics in the USA and in Europe. Contact Dermatitis. 2010;62(4):221-224. doi:10.1111/j.1600-0536.2009.01623.x
- Nguyen HL, Yiannias JA. Contact Dermatitis to Medications and Skin Products. Clin Rev Allergy Immunol. 2019;56(1):41-59. doi:10.1007/s12016-018-8705-0
- Hauksson I, Pontén A, Isaksson M, Hamada H, Engfeldt M, Bruze M. Formaldehyde in cosmetics in patch tested dermatitis patients with and without contact allergy to formaldehyde. Contact Dermatitis. 2016;74(3):145-151. doi:10.1111/cod.12493
- Giménez-Arnau AM, Deza G, Bauer A, et al. Contact allergy to preservatives: ESSCA* results with the baseline series, 2009-2012. J Eur Acad Dermatol Venereol. 2017;31(4):664-671. doi:10.1111/jdv.14063
- de Groot AC, Flyvholm MA, Lensen G, Menné T, Coenraads PJ. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermatitis. 2009;61(2):63-85. doi:10.1111/j.1600-0536.2009.01582.x
- Nassan FL, Mínguez-Alarcón L, Williams PL, et al. Urinary triclosan concentrations and semen quality among men from a fertility clinic. Environ Res. 2019;177:108633. doi:10.1016/j.envres.2019.108633
- Jurewicz J, Radwan M, Wielgomas B, et al. Environmental levels of triclosan and male fertility. Environ Sci Pollut Res Int. 2018;25(6):5484-5490. doi:10.1007/s11356-017-0866-5
- Zhu W, Zhou W, Huo X, et al. Triclosan and Female Reproductive Health: A Preconceptional Cohort Study. Epidemiology. 2019;30 Suppl 1:S24-S31. doi:10.1097/EDE.0000000000001011
- Cai S, Zhu J, Sun L, et al. Association Between Urinary Triclosan With Bone Mass Density and Osteoporosis in US Adult Women, 2005‒ J Clin Endocrinol Metab. 2019;104(10):4531-4538. doi:10.1210/jc.2019-00576
- Skarha J, Mínguez-Alarcón L, Williams PL, et al. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ Int. 2019;122:256-262. doi:10.1016/j.envint.2018.11.015
- Bever CS, Rand AA, Nording M, et al. Effects of triclosan in breast milk on the infant fecal microbiome. Chemosphere. 2018;203:467-473. doi:10.1016/j.chemosphere.2018.03.186
- Wolff MS, Teitelbaum SL, McGovern K, et al. Environmental phenols and pubertal development in girls. Environ Int. 2015;84:174-180. doi:10.1016/j.envint.2015.08.008
- Priyanka, Trivedi A, Maske P, Mote C, Dighe V. Gestational and lactational exposure to triclosan causes impaired fertility of F1 male offspring and developmental defects in F2 generation. Environ Pollut. 2020;257:113617. doi:10.1016/j.envpol.2019.113617
- He K, Huang J, Lagenaur CF, Aizenman E. Methylisothiazolinone, a neurotoxic biocide, disrupts the association of SRC family tyrosine kinases with focal adhesion kinase in developing cortical neurons. J Pharmacol Exp Ther. 2006;317(3):1320-1329. doi:10.1124/jpet.106.103044
- Bauman TM, Sheinbein DM. Chronic Urticaria Associated With Methylisothiazolinone Type IV Hypersensitivity. JAMA Dermatol. 2018;154(5):622-623. doi:10.1001/jamadermatol.2017.6161
- Dararattanaroj W, Pootongkam S, Rojanawatsirivej N, Wongpiyabovorn J. Patterns and risk factors of causative contact allergens in Thai adult patients with contact dermatitis. Asian Pac J Allergy Immunol. 2017;35(1):27-32. doi:10.12932/AP0757
- Scherrer MA, Rocha VB. Increasing trend of sensitization to Methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). An Bras Dermatol. 2014;89(3):527-528. doi:10.1590/abd1806-4841.20142852
- Herman A, Aerts O, de Montjoye L, Tromme I, Goossens A, Baeck M. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33(2):267-276. doi:10.1111/jdv.15267
- Marrero-Alemán G, Saavedra Santana P, Liuti F, Hernández N, López-Jiménez E, Borrego L. The Role of Cleaning Products in Epidemic Allergic Contact Dermatitis to Methylchloroisothiazolinone/Methylisothiazolinone. Dermatitis. 2018;29(2):77-80. doi:10.1097/DER.0000000000000352
- de Unamuno B, Zaragoza Ninet V, Sierra C, de la Cuadra J. Descriptive study of sensitization to methylchloroisothiazolinone and methylisothiazolinone in a skin allergy unit. Actas Dermosifiliogr. 2014;105(9):854-859. doi:10.1016/j.ad.2014.02.005
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of Contact Allergens in Personal Care Products for Babies and Children. Dermatitis. 2018;29(2):81-84. doi:10.1097/DER.0000000000000348
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: Retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84(4):965-976. doi:10.1016/j.jaad.2020.07.059
- Bilal M, Iqbal HMN. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals - A review. Sci Total Environ. 2019;670:555-568. doi:10.1016/j.scitotenv.2019.03.261
- Kabacoff BL, Douglass ML, Rosenberg IE, et al. Formation of nitrosamines in non-ionic and anionic emulsions in the presence and absence of inhibitors. IARC Sci Publ. 1984;(57):347-352.
- Lim DS, Lim SK, Kim MK, et al. Formation and inhibition of N-nitrosodiethanolamine in cosmetics under pH, temperature, and fluorescent, ultraviolet, and visual light. J Toxicol Environ Health A. 2018;81(9):241-253. doi:10.1080/15287394.2018.1440172
- Lijinsky W, Reuber MD, Manning WB. Potent carcinogenicity of nitrosodiethanolamine in rats. Nature. 1980;288(5791):589-590. doi:10.1038/288589a0
- Hecht SS. Approaches to cancer prevention based on an understanding of N-nitrosamine carcinogenesis. Proc Soc Exp Biol Med. 1997;216(2):181-191. doi:10.3181/00379727-216-44168
- Ito N, Fukushima S, Tsuda H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit Rev Toxicol. 1985;15(2):109-150. doi:10.3109/10408448509029322
- Ham J, Lim W, You S, Song G. Butylated hydroxyanisole induces testicular dysfunction in mouse testis cells by dysregulating calcium homeostasis and stimulating endoplasmic reticulum stress. Sci Total Environ. 2020;702:134775. doi:10.1016/j.scitotenv.2019.134775
- Park S, Lee JY, Lim W, You S, Song G. Butylated Hydroxyanisole Exerts Neurotoxic Effects by Promoting Cytosolic Calcium Accumulation and Endoplasmic Reticulum Stress in Astrocytes [published correction appears in J Agric Food Chem. 2019 Oct 9;67(40):11277]. J Agric Food Chem. 2019;67(34):9618-9629. doi:10.1021/acs.jafc.9b02899
- Matsuoka A, Matsui M, Miyata N, Sofuni T, Ishidate M Jr. Mutagenicity of 3-tert-butyl-4-hydroxyanisole (BHA) and its metabolites in short-term tests in vitro. Mutat Res. 1990;241(2):125-132. doi:10.1016/0165-1218(90)90115-i
- Li X, Cao S, Mao B, et al. Effects of butylated hydroxyanisole on the steroidogenesis of rat immature Leydig cells. Toxicol Mech Methods. 2016;26(7):511-519. doi:10.1080/15376516.2016.1202367
- Pop A, Kiss B, Loghin F. Endocrine disrupting effects of butylated hydroxyanisole (BHA – E320). Clujul Med. 2013;86(1):16-20.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105(9):822-832. doi:10.1016/j.ad.2013.12.018
- Nguyen HL, Yiannias JA. Contact Dermatitis to Medications and Skin Products. Clin Rev Allergy Immunol. 2019;56(1):41-59. doi:10.1007/s12016-018-8705-0
- Saint-Mezard P, Rosieres A, Krasteva M, et al. Allergic contact dermatitis. Eur J Dermatol. 2004;14(5):284-295.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Hair dye dermatitis and p-phenylenediamine contact sensitivity: A preliminary report. Indian Dermatol Online J. 2015;6(4):241-246. doi:10.4103/2229-5178.160253
- Uter W, Lessmann H, Geier J, Schnuch A. Contact allergy to hairdressing allergens in female hairdressers and clients--current data from the IVDK, 2003-2006. J Dtsch Dermatol Ges. 2007;5(11):993-1001. doi:10.1111/j.1610-0387.2007.06511.x
- Adams RM, Maibach HI. A five-year study of cosmetic reactions.J Am Acad Dermatol. 1985;13(6):1062-1069. doi:10.1016/s0190-9622(85)70258-7
- Farsani TT, Jalian HR, Young LC. Chemical leukoderma from hair dye containing para-phenylenediamine. Dermatitis. 2012;23(4):181-182. doi:10.1097/DER.0b013e318260d5cd
- Eiermann HJ, Larsen W, Maibach HI, Taylor JS. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6(5):909-917. doi:10.1016/s0190-9622(82)70080-5
- Handa S, Mahajan R, De D. Contact dermatitis to hair dye: an update. Indian J Dermatol Venereol Leprol. 2012;78(5):583-590. doi:10.4103/0378-6323.100556
- Seo JA, Bae IH, Jang WH, et al. Hydrogen peroxide and monoethanolamine are the key causative ingredients for hair dye-induced dermatitis and hair loss. J Dermatol Sci. 2012;66(1):12-19. doi:10.1016/j.jdermsci.2011.12.015
- Gentian Violet. In: Drugs and Lactation Database (LactMed). Bethesda (MD): National Library of Medicine (US); November 16, 2020.
- Reeder MJ. Allergic Contact Dermatitis to Fragrances. Dermatol Clin. 2020;38(3):371-377. doi:10.1016/j.det.2020.02.009
- National Toxicology Program, US Department of Health and Human Services. Report on Carcinogens, Fourteenth Edition. RoC Profile: Ethylene Oxide (nih.gov)
- National Toxicology Program, US Department of Health and Human Services. Report on Carcinogens, Fourteenth Edition. RoC Profile: Dioxane (nih.gov)
- Kim MK, Kim KB, Yoon S, Kim HS, Lee BM. Risk assessment of unintentional phthalates contaminants in cosmetics. Regul Toxicol Pharmacol. 2020;115:104687. doi:10.1016/j.yrtph.2020.104687
- Bocca B, Pino A, Alimonti A, Forte G. Toxic metals contained in cosmetics: a status report. Regul Toxicol Pharmacol. 2014;68(3):447-467. doi:10.1016/j.yrtph.2014.02.003
- Saadatzadeh A, Afzalan S, Zadehdabagh R, et al. Determination of heavy metals (lead, cadmium, arsenic, and mercury) in authorized and unauthorized cosmetics. Cutan Ocul Toxicol. 2019;38(3):207-211. doi:10.1080/15569527.2019.1590389
- Chang CJ, Tu YK, Chen PC, Yang HY. Occupational Exposure to Talc Increases the Risk of Lung Cancer: A Meta-Analysis of Occupational Cohort Studies. Can Respir J. 2017;2017:1270608. doi:10.1155/2017/1270608
- Bakthavachalu P, Kannan SM, Qoronfleh MW. Food Color and Autism: A Meta-Analysis. Adv Neurobiol. 2020;24:481-504. doi:10.1007/978-3-030-30402-7_15
- Stevens LJ, Burgess JR, Stochelski MA, Kuczek T. Amounts of artificial food colors in commonly consumed beverages and potential behavioral implications for consumption in children. Clin Pediatr (Phila). 2014;53(2):133-140. doi:10.1177/0009922813502849
- Vojdani A, Vojdani C. Immune reactivity to food coloring. Altern Ther Health Med. 2015;21 Suppl 1:52-62.
- Willett, WC et al. 1993. “Intake of Trans Fatty Acids and Risk of Coronary Heart Disease among Women.” Lancet 341(8845):581–585.
- Hu, FB et al. 1997. “Dietary Fat Intake and the Risk of Coronary Heart Disease in Women.” N Engl J Med 337(21):1491–1499.
- Oh, K et al. 2005. “Dietary Fat Intake and Risk of Coronary Heart Disease in Women: 20 Years of Follow-Up of the Nurses’ Health Study.” Am J Epidemiol 161(7):672–679.
- Ascherio, A et al. 1996. “Dietary Fat and Risk of Coronary Heart Disease in Men: Cohort Follow Up Study in the United States.” BMJ 313(7049):84–90.
- Oomen, CM et al. 2001. “Association between Trans Fatty Acid Intake and 10-Year Risk of Coronary Heart Disease in the Zutphen Elderly Study: A Prospective Population-Based Study.” Lancet 357(9258):746–751.
- Mozaffarian, D et al. 2006. “Trans Fatty Acids and Cardiovascular Disease.” N Engl J Med 354(15):1601–1613.
- Bendsen, NT et al. 2011. “Consumption of Industrial and Ruminant Trans Fatty Acids and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Cohort Studies.” Eur J Clin Nutr 65(7):773–783.
- De Souza, RJ et al. 2015. “Intake of Saturated and Trans Unsaturated Fatty Acids and Risk of All Cause Mortality, Cardiovascular Disease, and Type 2 Diabetes: Systematic Review and Meta-Analysis of Observational Studies.” BMJ 351:h3978.
- Melanson SF, Lewandrowski EL, Flood JG, Lewandrowski KB. Measurement of organochlorines in commercial over-the-counter fish oil preparations: implications for dietary and therapeutic recommendations for omega-3 fatty acids and a review of the literature. Arch Pathol Lab Med. 2005;129(1):74-77. doi:10.5858/2005-129-74-MOOICO
- Rawn DF, Breakell K, Verigin V, Nicolidakis H, Sit D, Feeley M. Persistent organic pollutants in fish oil supplements on the Canadian market: polychlorinated biphenyls and organochlorine insecticides. J Food Sci. 2009;74(1):T14-T19. doi:10.1111/j.1750-3841.2008.01020.x
- Fernandes AR, Rose M, White S, Mortimer DN, Gem M. Dioxins and polychlorinated biphenyls (PCBs) in fish oil dietary supplements: occurrence and human exposure in the UK. Food Addit Contam. 2006;23(9):939-947. doi:10.1080/02652030600660827
- Jacobs MN, Santillo D, Johnston PA, Wyatt CL, French MC. Organochlorine residues in fish oil dietary supplements: comparison with industrial grade oils. Chemosphere. 1998;37(9-12):1709-1721. doi:10.1016/s0045-6535(98)00236-7
- Martí M, Ortiz X, Gasser M, Martí R, Montaña MJ, Díaz-Ferrero J. Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere. 2010;78(10):1256-1262. doi:10.1016/j.chemosphere.2009.12.038
- Ashley JT, Ward JS, Anderson CS, et al. Children's daily exposure to polychlorinated biphenyls from dietary supplements containing fish oils. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(3):506-514. doi:10.1080/19440049.2012.753161
- Levine KE, Levine MA, Weber FX, Hu Y, Perlmutter J, Grohse PM. Determination of mercury in an assortment of dietary supplements using an inexpensive combustion atomic absorption spectrometry technique. J Autom Methods Manag Chem. 2005;2005(4):211-216. doi:10.1155/JAMMC.2005.211
- Figueiredo A, Costa IM, Fernandes TA, Gonçalves LL, Brito J. Food Supplements for Weight Loss: Risk Assessment of Selected Impurities. Nutrients. 2020;12(4):954. Published 2020 Mar 30. doi:10.3390/nu12040954
- Rehman S, Adnan M, Khalid N, Shaheen L. Calcium supplements: an additional source of lead contamination. Biol Trace Elem Res. 2011;143(1):178-187. doi:10.1007/s12011-010-8870-3
- Ćwieląg-Drabek M, Piekut A, Szymala I, et al. Health risks from consumption of medicinal plant dietary supplements. Food Sci Nutr. 2020;8(7):3535-3544. Published 2020 May 19. doi:10.1002/fsn3.1636
- Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014;2014:840547. doi:10.1155/2014/840547
- Rzymski P, Tomczyk K, Rzymski P, Poniedziałek B, Opala T, Wilczak M. Impact of heavy metals on the female reproductive system. Ann Agric Environ Med. 2015;22(2):259-264. doi:10.5604/12321966.1152077
- Al Osman M, Yang F, Massey IY. Exposure routes and health effects of heavy metals on children. Biometals. 2019;32(4):563-573. doi:10.1007/s10534-019-00193-5
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. doi:10.1155/2012/460508
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609-662. doi:10.1080/10408440600845619
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005;5(2):153-160. doi:10.1385/ct:5:2:153
- Fischer LJ, Seegal RF, Ganey PE, Pessah IN, Kodavanti PR. Symposium overview: toxicity of non-coplanar PCBs. Toxicol Sci. 1998;41(1):49-61. doi:10.1006/toxs.1997.2386
- Wahlang B, Jin J, Hardesty JE, et al. Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food Chem Toxicol. 2019;129:64-76. doi:10.1016/j.fct.2019.04.007
- Pěnčíková K, Svržková L, Strapáčová S, et al. In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut. 2018;237:473-486. doi:10.1016/j.envpol.2018.02.067
- Knerr S, Schrenk D. Carcinogenicity of "non-dioxinlike" polychlorinated biphenyls. Crit Rev Toxicol. 2006;36(9):663-694. doi:10.1080/10408440600845304
- Nogueira CM, de Azevedo WM, Dagli ML, et al. Titanium dioxide induced inflammation in the small intestine. World J Gastroenterol. 2012;18(34):4729-4735. doi:10.3748/wjg.v18.i34.472
- Partridge D, Lloyd KA, Rhodes JM, Walker AW, Johnstone AM, Campbell BJ. Food additives: Assessing the impact of exposure to permitted emulsifiers on bowel and metabolic health - introducing the FADiets study. Nutr Bull. 2019;44(4):329-349. doi:10.1111/nbu.12408
- Viennois E, Chassaing B. Consumption of Select Dietary Emulsifiers Exacerbates the Development of Spontaneous Intestinal Adenoma. Int J Mol Sci. 2021;22(5):2602. Published 2021 Mar 5. doi:10.3390/ijms22052602
- Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66(8):1414-1427. doi:10.1136/gutjnl-2016-313099
- Georgia State University. "Widely used food additives promotes colitis, obesity and metabolic syndrome, shows study of emulsifiers." ScienceDaily. ScienceDaily, 25 February 2015.
- Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohns Colitis. 2013;7(4):338-341. doi:10.1016/j.crohns.2013.01.004
- Holder, M.K., Peters, N.V., Whylings, J. et al. Dietary emulsifiers consumption alters anxiety-like and social-related behaviors in mice in a sex-dependent manner. Sci Rep 9, 172 (2019). https://doi.org/10.1038/s41598-018-36890-3
- Rinninella E, Cintoni M, Raoul P, Gasbarrini A, Mele MC. Food Additives, Gut Microbiota, and Irritable Bowel Syndrome: A Hidden Track. Int J Environ Res Public Health. 2020;17(23):8816. Published 2020 Nov 27. doi:10.3390/ijerph17238816
Search the blog
Article Categories
- All Articles (95)
- Rating Charts (1)
- Beauty & Skincare (17)
- FAQ (0)
- Hair Care (9)
- Health & Wellness (12)
- Anti-Aging (4)
- Kid's Health (0)
- Makeup (2)
- Men's Health (2)
- Oral Care (3)
- Sunscreen (7)
- Skin Tools & Treatments (10)
- Supplements (26)
- Videos (0)





